

In order to react flexibly in future to customer requirements, Recipharm commissioned the MCP project team at MULTIVAC with the task of developing an upgradable solution for the automated bundling and cartonning of eyedrop ampoules with an additional pack leaflet. The modular concept, which appears very simple in summary, was a real challenge for a variety of reasons for the experienced packaging specialists at MULTIVAC.
Recipharm Kaysersberg Pharmaceuticals S.A.S. was founded in 1935 in a chemist’s shop, and it was part of Novartis AG from 2008 before being sold to the Swedish contract manufacturer Recipharm. The company now employs around 330 staff. Some 90 percent of the turnover is generated through exports to 55 countries. The main area of business is the filling and packing of pharmaceutical products by means of Blow-Fill-Seal (BFS) technology. A total of 10,330 m² of production area is available in the French town of Kaysersberg. In 2017, around 400 million pharmaceutical doses were packed in some 15 million batches. Thanks to a new packaging line, the available capacity is due to be increased to 600 million doses in 2018.
Recipharm envisaged an automated solution for this, one which “could grow in various expansion stages with our constantly rising requirements, and with which we can manage the wide range of customer orders flexibly and efficiently,” says Yves Buelens, General Manager at Recipharm, summarising the objectives of the project.
The new packaging solution was intended to be designed in such a way that, depending on the particular customer requirement, the ampoules could be packaged in different ways - either in a folding carton or alternatively in a thermoformed pack made of aluminium multi-layer film, which is subsequently secondary packaged in a folding carton. In addition to direct printing of the ampoules, the concept also had to include the possibility of inline labelling as a second version of product marking - as well as a further expansion stage, which enables the products to be packed in plastic boxes as “product for stock”.
The first phase of the project implementation was successfully completed in February 2018. In addition to the product infeed, alignment and converging systems, the packaging solution now also includes the equipment for loading the folding cartons as well as a variety of quality inspection solutions. “When the project began, the most intensive challenge apart from the limited space in the production hall was the fact that the filling process for eyedrops is a continuous process, which must not be interrupted, if at all possible. When developing a suitable solution, it was also therefore necessary to plan a buffer function for the filled ampoules,” says Karl-Heinz Weigele, Project Manager for the MCP Medical and Pharmaceutical Division, getting straight to the point on the very difficult demands of the project.


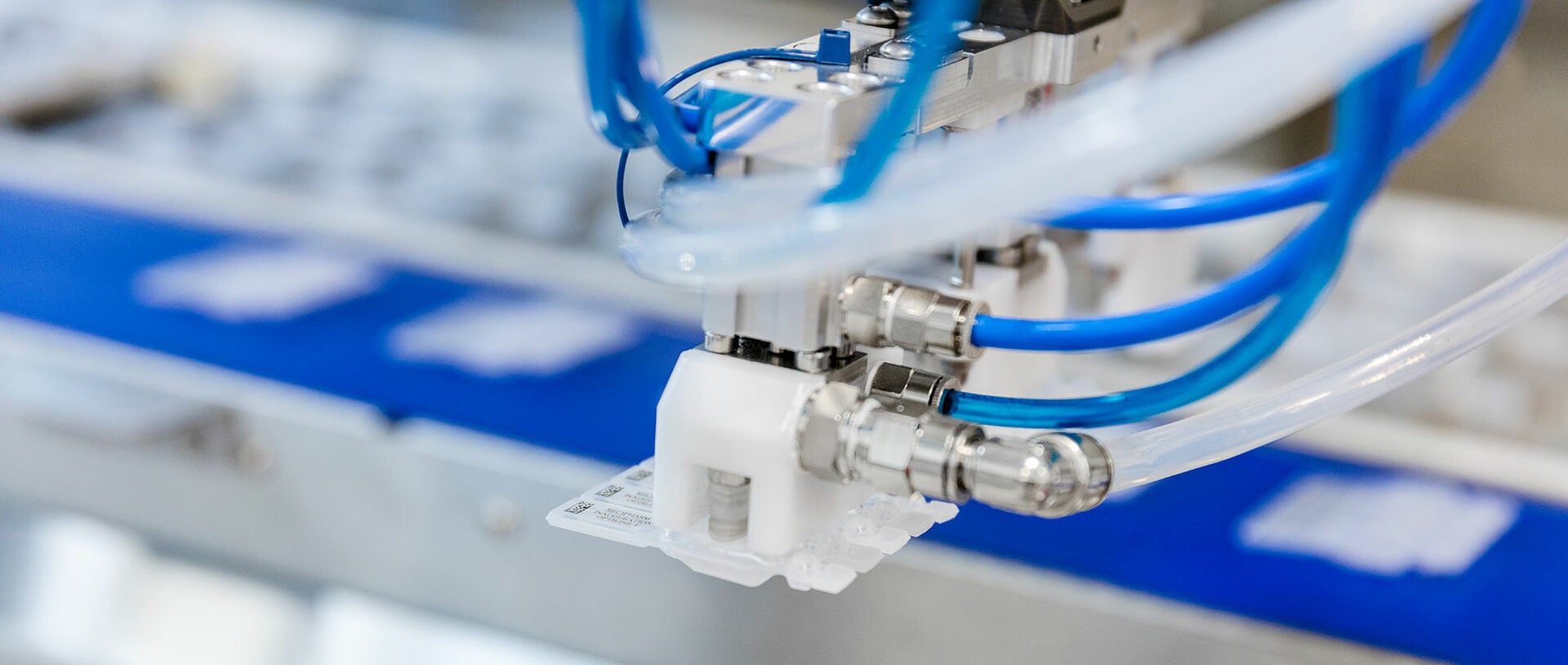
Recipharm uses the so-called Blow-Fill-Seal technology for filling the ampoules. The filled ampoules are printed in strips of 4 or 5 and then fed via a transport conveyor to the adjoining packaging line. A MULTIVAC vision system, which enables a wide range of detection and inspection tasks to be performed, checks the printed data, captures the orientation and reports the position to a handling module via the IPC control. The two robot systems grip the products one after the other from the conveyor, and then they orientate and place them very precisely in a carrier system. If the print data is not correct, the products are not gripped by the robot. These products drop into a waste container. If this occurrence happens three consecutive times, the system stops immediately.
Thanks to the precise transfer of the ampoules from the conveyor to an opposite-running carrier system, the ampoules are now accurately aligned for the subsequent processes such as labelling, thermoforming packaging, and cartonning. The role of the carrier system is therefore to convert the products from an uncontrolled infeed to a controlled state for further processing. “Even though the line components of thermoforming packaging and inline labelling are only due to be integrated into the complete solution at a later date, we have already taken them into account in our line concept. In order to achieve this, the packaging solution was equipped with the corresponding interfaces, and preparations have already been made to implement the required process stages,” adds Karl-Heinz Weigele.
The loaded carrier is then transported away for further processing via transport conveyors. “The current situation is that the strips of unpackaged plastic ampoules are placed into the folding cartons in different numbers of layers. As already mentioned, however, by Karl-Heinz Weigele, it is already planned to introduce in future the packing of the products in aluminium multi-layer film on a thermoforming packaging machine as a further packaging stage,” confirms Yves Buelens.
Here, the folding carton is erected and adhered with hot glue. The cartonning module itself is a system component, which was supplied by a third-party manufacturer and integrated by MULTIVAC into the line. Each carton is already pre-printed with a code, which is stored in the IPC of the H 242 handling module, so that it can be checked. The carton is only transported onwards, if the checking process shows that it is correct, and a dispenser subsequently dispenses the pack leaflet. If the code can not be read, or if it is missing completely, the carton is ejected into a waste container. If the code is legible but incorrect, the whole process is stopped immediately.
The pack leaflet is also pre-printed with a code, which is stored in the IPC of the H 242. The same scenario is repeated: if the checking process shows that the code is correct, the leaflet is placed in the carton by means of a dispensing arm. If the code can not be read, or if it is missing completely, the leaflet is ejected into a waste container. If the code is legible but incorrect, the whole process is again stopped immediately.


A further H 242 handling module loads the products automatically into the cartons. Two strips at once are picked up by the suction gripper and placed in the carton. The system is designed so flexibly, that it can handle many different product configurations. After the carton has been filled, it is closed automatically and sealed with hot glue. The cartons are then weighed. If any have the incorrect weight, they are ejected immediately. If required, the cartons can be bundled together by means of a wrapping machine as a final process stage. Either simple straps or stretch-wrapping are available for this process. A transport conveyor then takes the cartons to a box packer and palletising unit. Here the cartons are automatically packed into boxes, which are then closed and stacked on pallets.
The high level of flexibility in the MULTIVAC solution has been very well received by Recipharm. Thanks to its modular construction, the whole concept offers many possibilities for expansion of the system, and it is also easy to operate and service. “A further benefit is the fact that it is possible to compensate for micro-stops within the system, and the overall equipment effectiveness is increased by the integrated buffers,” explains Yves Buelens.
The buffer function has a special significance in this packaging solution. It was planned in from the start, and it is also important in the first project phase for the upstream machine, which must not stop if a downstream machine breaks down. “Small stops in automated solutions often cause significant losses in the effectiveness of the overall line,” as Karl-Heinz Weigele knows only too well.
At Recipharm there are two so-called buffer towers: the first tower is intended for the loaded carriers, while the second is for empty carriers. The first buffer tower is used, after the plastic ampoules have been loaded into the carriers. The filled carriers are buffered in case a downstream machine such as a labeller, thermoforming packaging machine, or box erector stops. If a loaded carrier gets to the buffer, an empty carrier is fed from the second buffer tower since it is designed as a closed system.
A so-called reject monitoring station is installed before the buffer tower for empty carriers. This makes sure the carriers, which get to the buffer tower, really are empty, i.e. without product. All the cavities of the carriers are checked by means of a light barrier. Should one strip of ampoules be present in the carrier, this carrier is taken out of the system by means of the reject station. It must then be emptied manually and fed back again by hand into the system.
The entire system is designed to be so open in its design and control technology that all the planned functions, or even some additional ones, can easily be integrated. The packaging solution can therefore be individually adapted to future requirements, and the system grows with them.
As an alternative to the current printing of the ampoules, it is also possible, for example, to dispense a label by means of an inline labeller. The very small products must however in this case be aligned and positioned precisely. “The high level of product positioning accuracy in the carriers was actually one of the greatest challenges for the handling module. However, we were able to successfully solve the problem of placing the products accurately, allowing this option to be implemented immediately when required,” says MULTIVAC’s project manager, Karl-Heinz Weigele.
In addition to this, the packaging procedure can be designed in such a way, that the currently unpackaged ampoules could also be packed in aluminium multi-layer packs on a thermoforming packaging machine. The MULTIVAC concept envisages, that the filled ampoules would be transported via a conveyor from the BFS system to the thermoforming packaging machine, where they would first be placed in the carrier by the handling module and labelled as they are lying. Then the labelled ampoules are automatically removed from the carrier and loaded into the pack cavities of the thermoforming packaging machine. Ther,e the strips are packed before being picked up automatically and placed in the carrier, which transports them to the packaging station, where they are automatically packed into the cartons.
In the case of the “product for stock” solution, the printed products are fed by means of a transport conveyor to the handling module, where they are placed in plastic boxes. These boxes then go into storage or for manual packing. This option was particularly important for Recipharm at the concept stage, since the market sometimes requires the ampoules to undergo quality control in the form of a leak test, which can only take place after a certain time has elapsed from the filling of the eyedrops. This means the ampoules in their plastic boxes must be stored in a warehouse at defined temperature and humidity conditions. After the ampoules have been successfully tested, they are fed back again into the packaging procedure.
One variant of “product for stock” is the subsequent manual packing of small batches. Here, the printed ampoules are also placed in plastic boxes by means of a handling module, after they have been filled. The content of the box is handled manually as required - either being packed by hand in small batches or stored for later quality tests.

Another aspect, which had to be taken into account during the development of the MULTIVAC solution, was secure line clearance, since the different production batches must be kept separate at a contract packer such as Recipharm. “The integration of inspection solutions in the line was also therefore extremely important for us,” emphasizes Yves Buelens. A colour-marked carrier is used for monitoring line clearance. The next batch can only be started, after this carrier has run once through the complete system, and if all the buffers have been emptied.
It was not a problem for MULTIVAC to provide reliable linking and control of all the line components, even those from third-party suppliers - “despite the interfaces for the scanners, which check the 2D codes on the leaflets and cartons, being significantly more extensive than envisaged, since they have to capture the operating status in many versions, such as legibility, correctness, illegibility, and non-presence etc,” says Karl-Heinz Weigele, summarising the requirements regarding the interfaces with third-party components.
Overall the solution fully meets the expectations of Recipharm. “Of course MULTIVAC’s concept seems from today’s perspective rather oversized, but we are already thinking about tomorrow. This is because we want to be fully equipped for future requirements - with a future-proofed solution, which we can expand successively with additional functions,” says Yves Buelens in summary.
07.06.2018