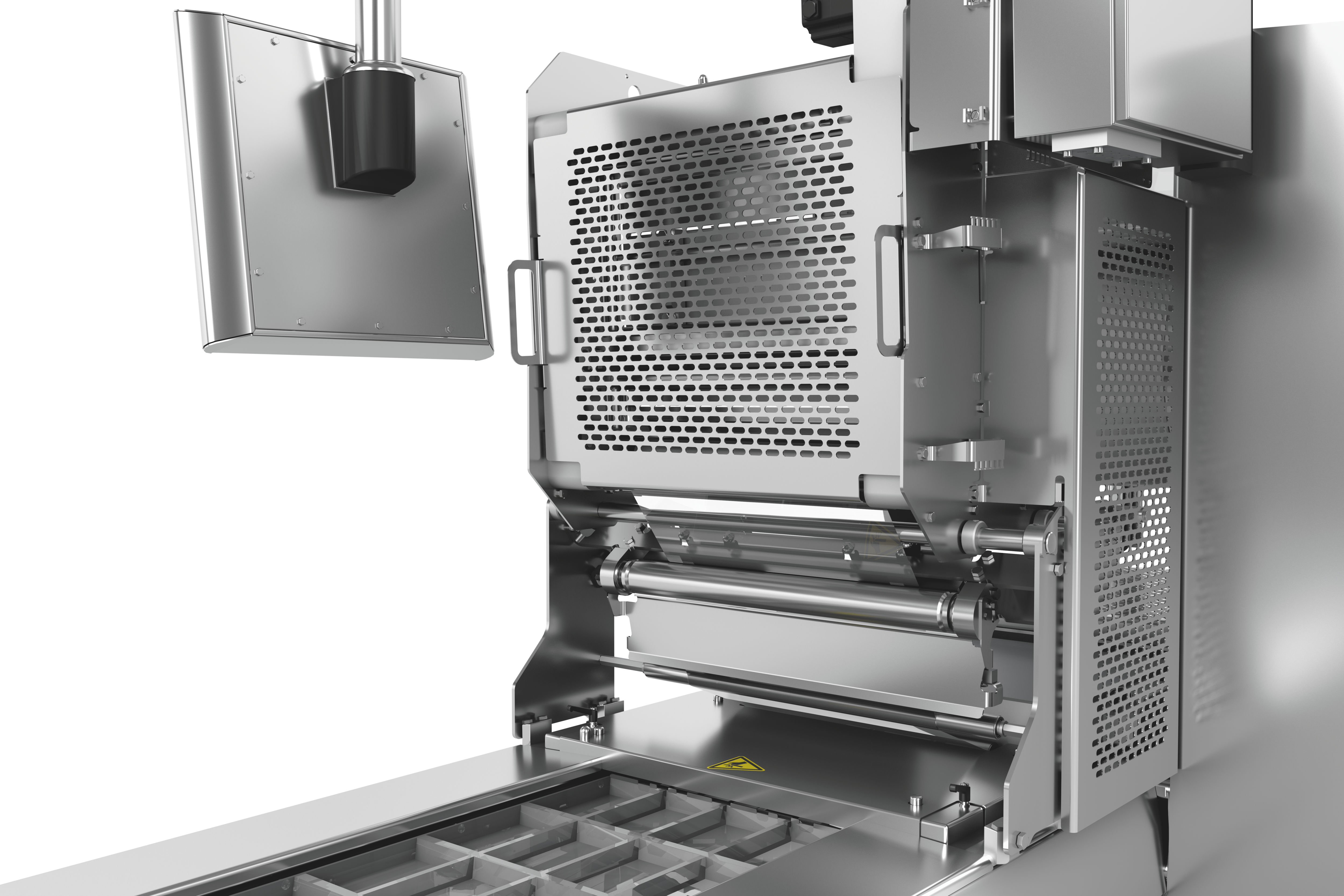
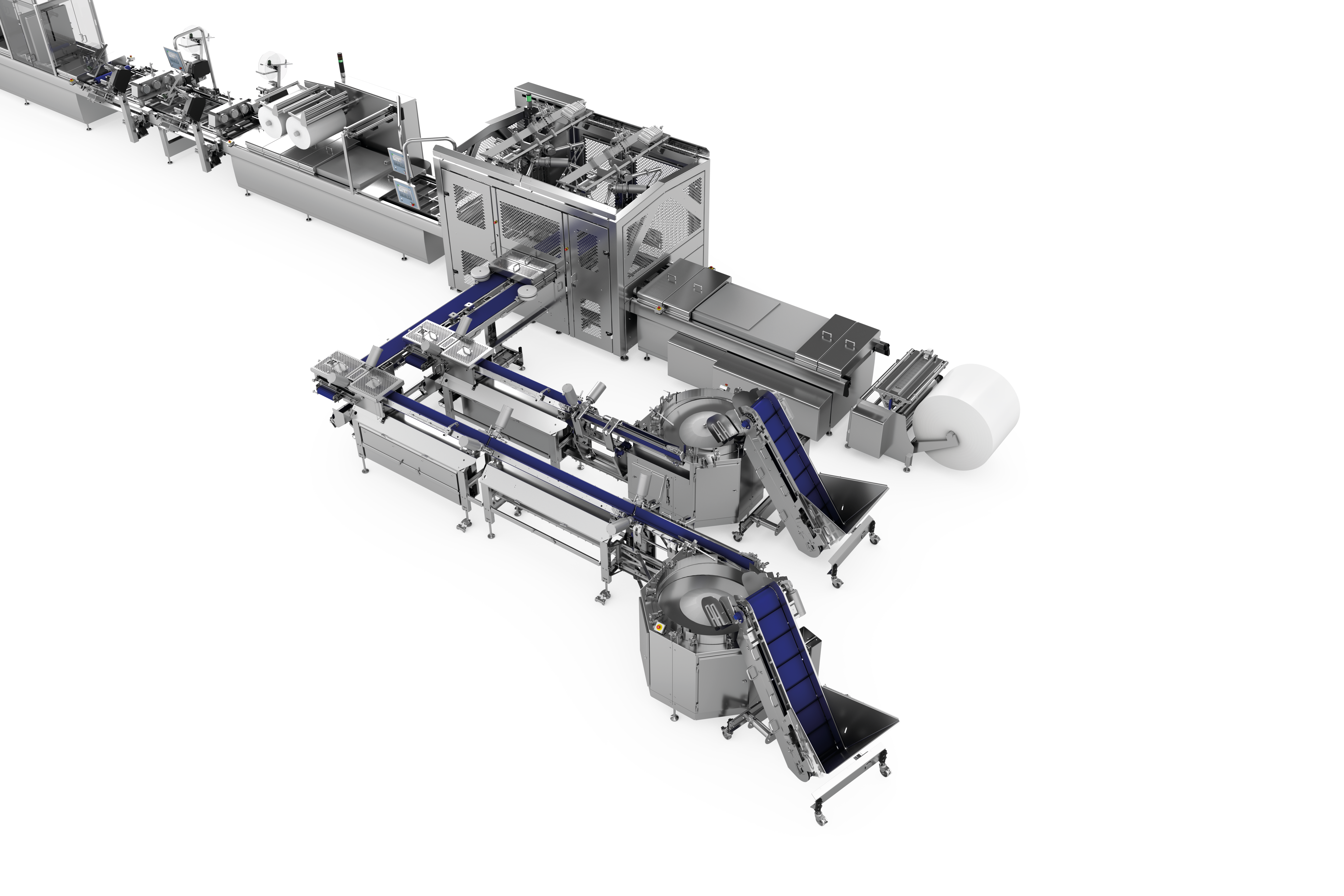
Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
גמישות ואמינות בתיוג וסימון של אריזות תרמופורמיות
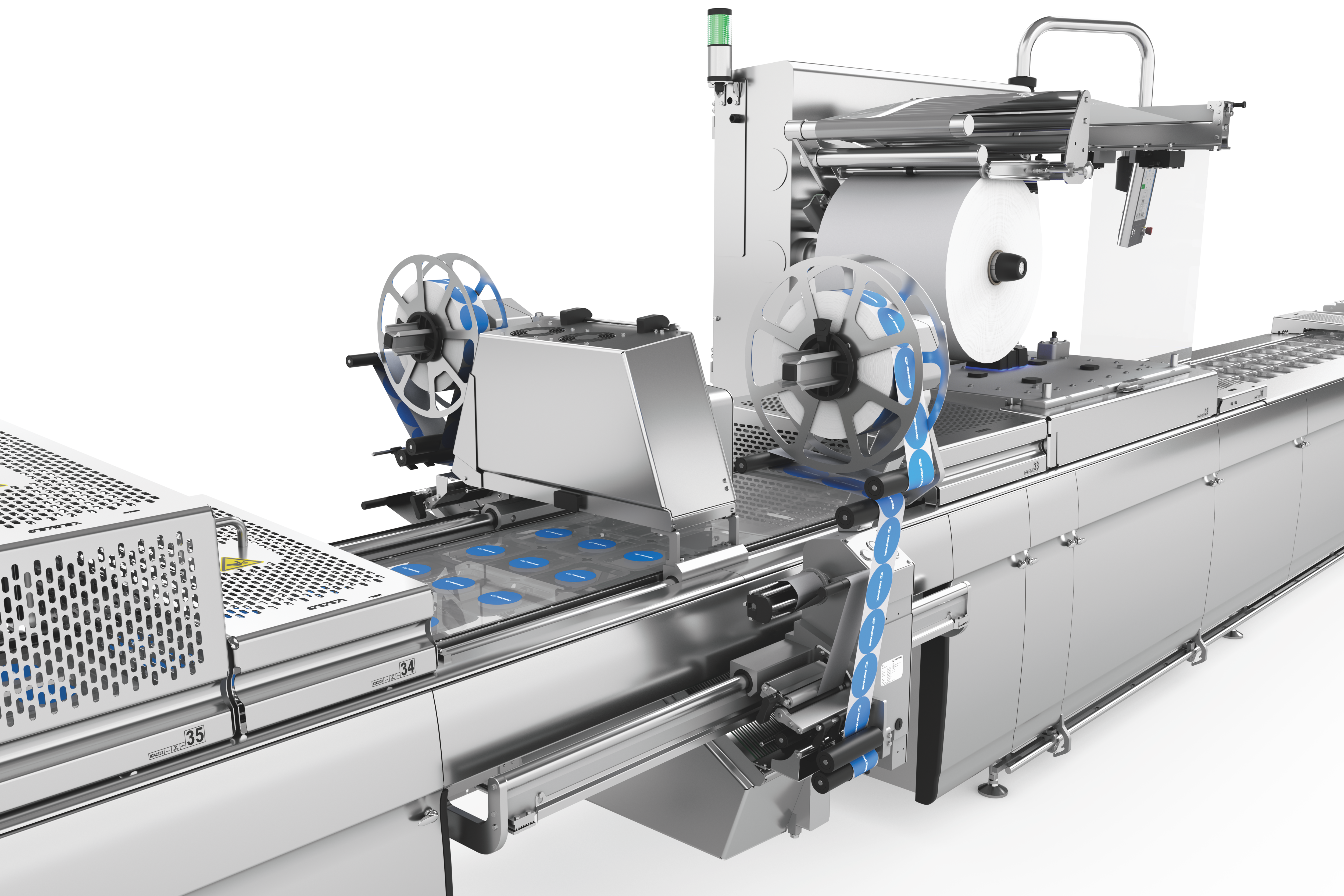
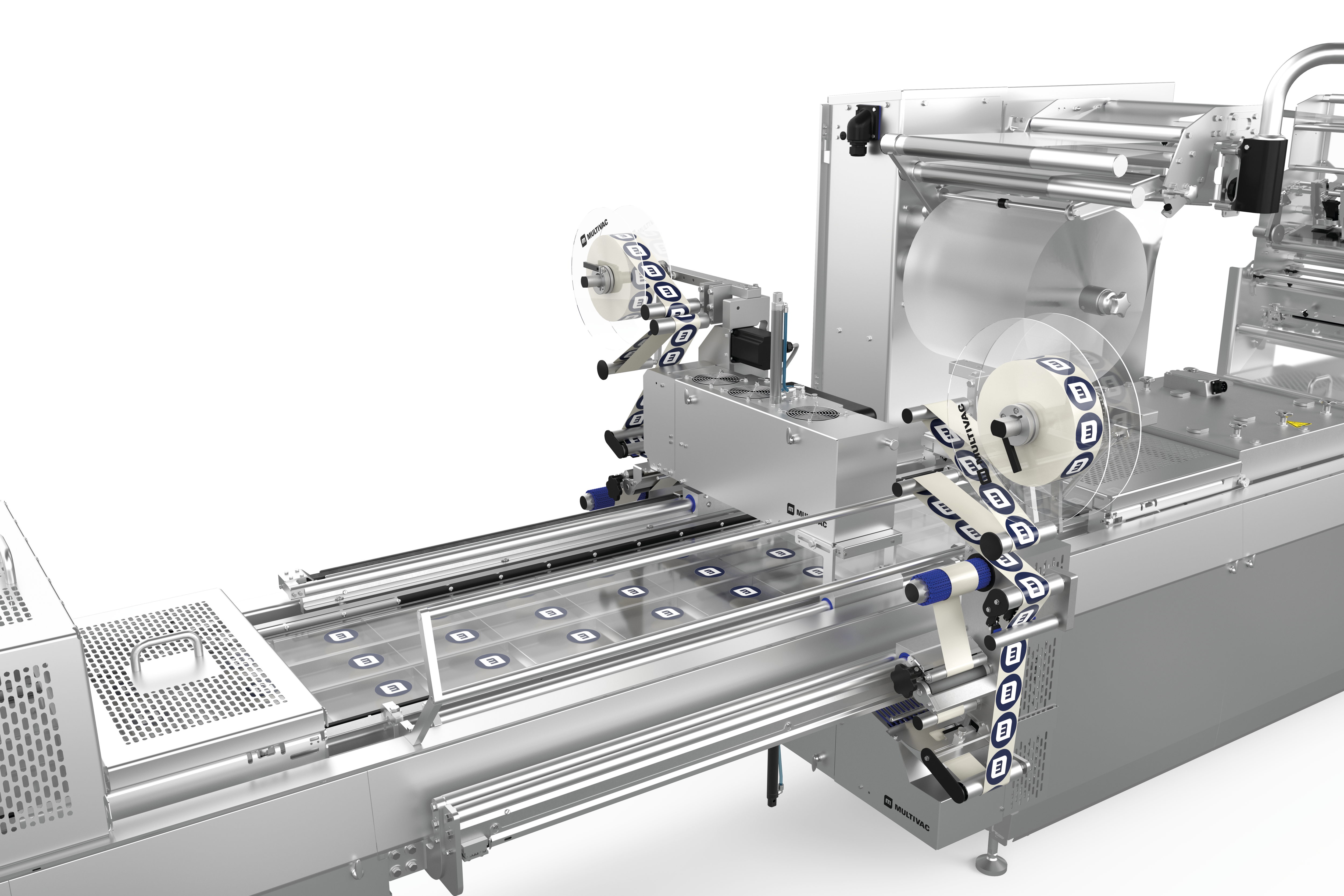
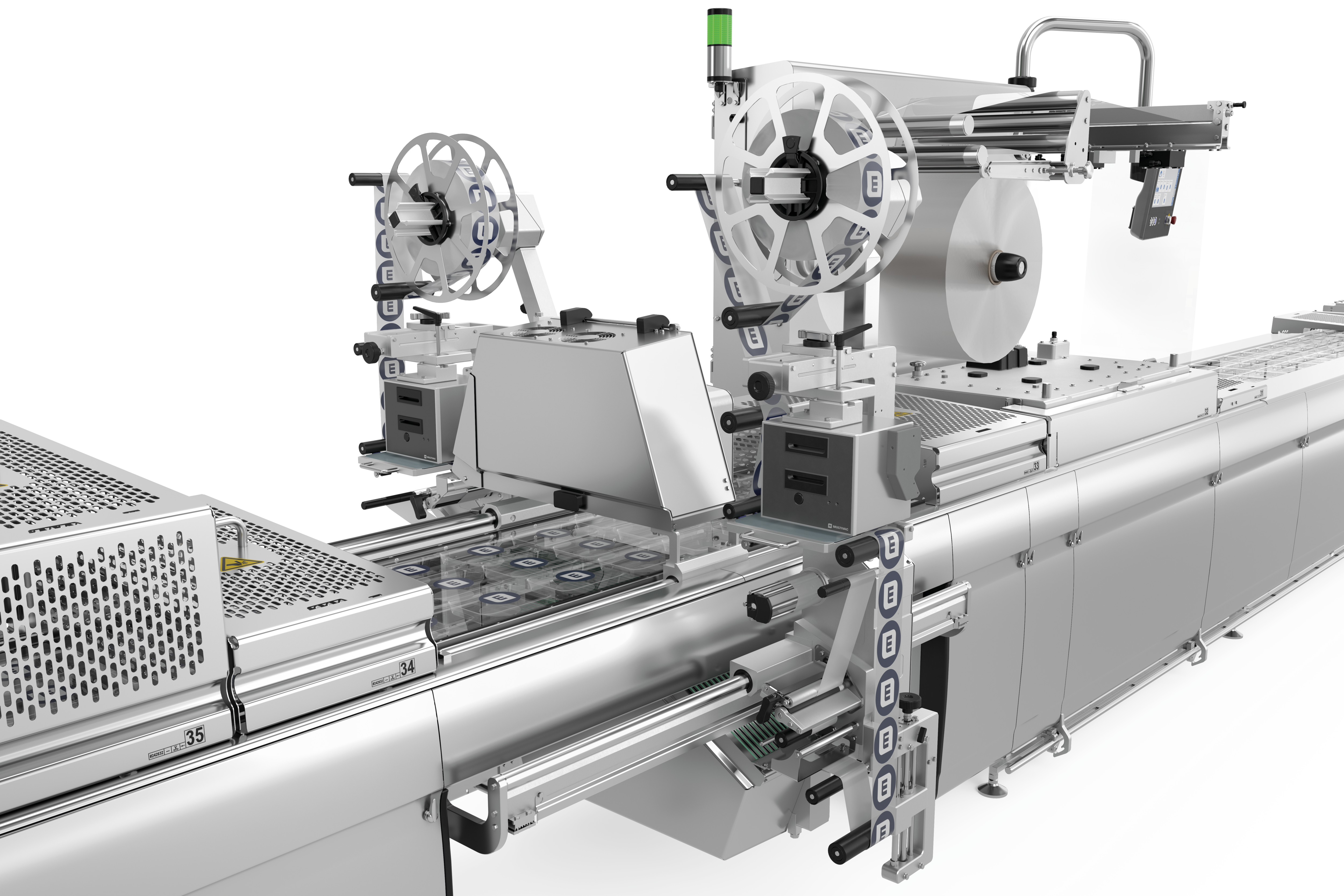
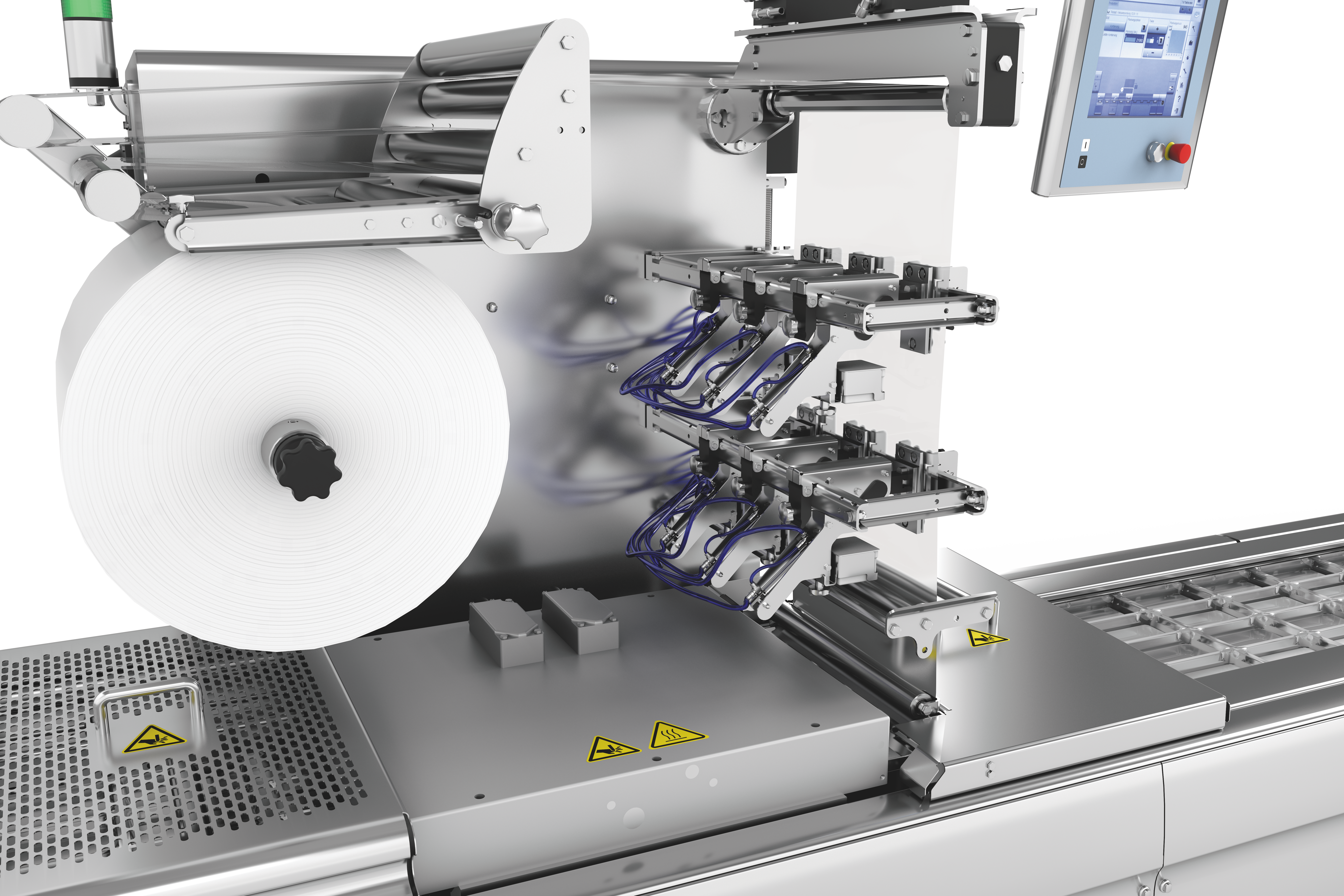
לסימון אריזות תרמופורמיות, אנו מציעים לך שני פתרונות תיוג הניתנים להתאמה אישית, גמישים וניתנים לשילוב מלא עם תוויות לרוחב ולאורך היריעה . מכונות התיוג ומדפסות היריעה הישירות נשלטות באופן מרכזי באמצעות HMI (ממשק מכונה-אנוש). נשמח לייעץ לכם על בחירת הפורמטים והחומרים.
יעילות וחסכוניות
דרישות השוק גדלות ללא הרף. חברות צריכות לעמוד בקצב ההתפתחויות וציפיות השוק כדי לשמור על מעמדן בעתיד. החלטות השקעה בנות קיימא מבחינה כלכלית צריכות להיות בטוחות לעתיד. זה כולל גם תיוג של אריזות thermoforming. זמני המרה ארוכים, למשל, משפיעים לרעה על יעילות הציוד. יתר על כן, אם רכיבי קו בודדים אינם פועלים כיחידה אחת אחידה, התהליך כולו ייפגע.
סיפורי הצלחה
 Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
Potato specialities securely packed and protected
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
למד עוד
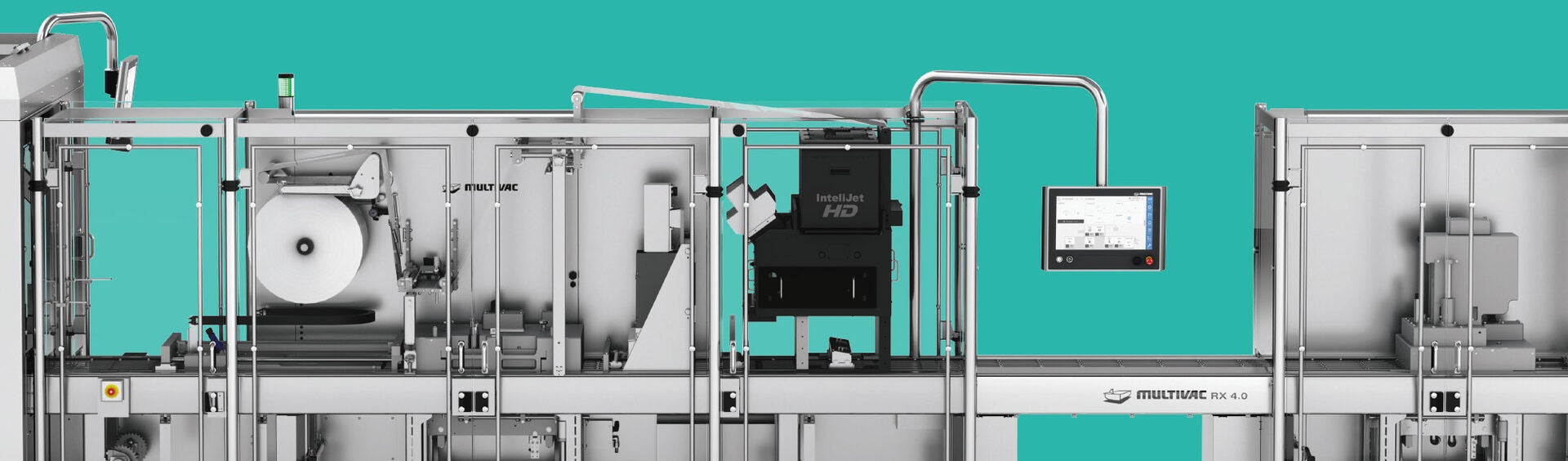
Digital printing systems fulfil challenging pack marking requirements
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
למד עוד