Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
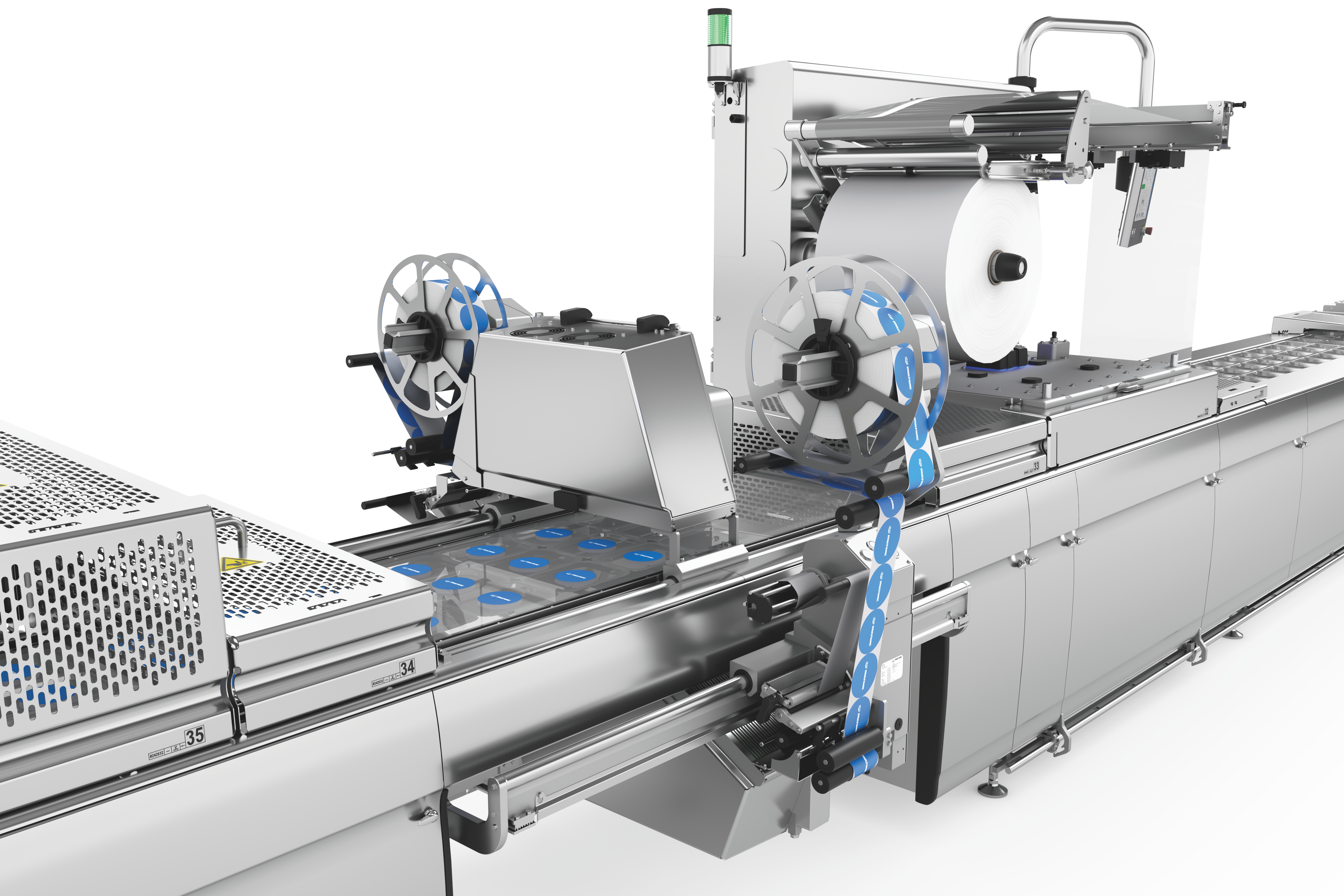
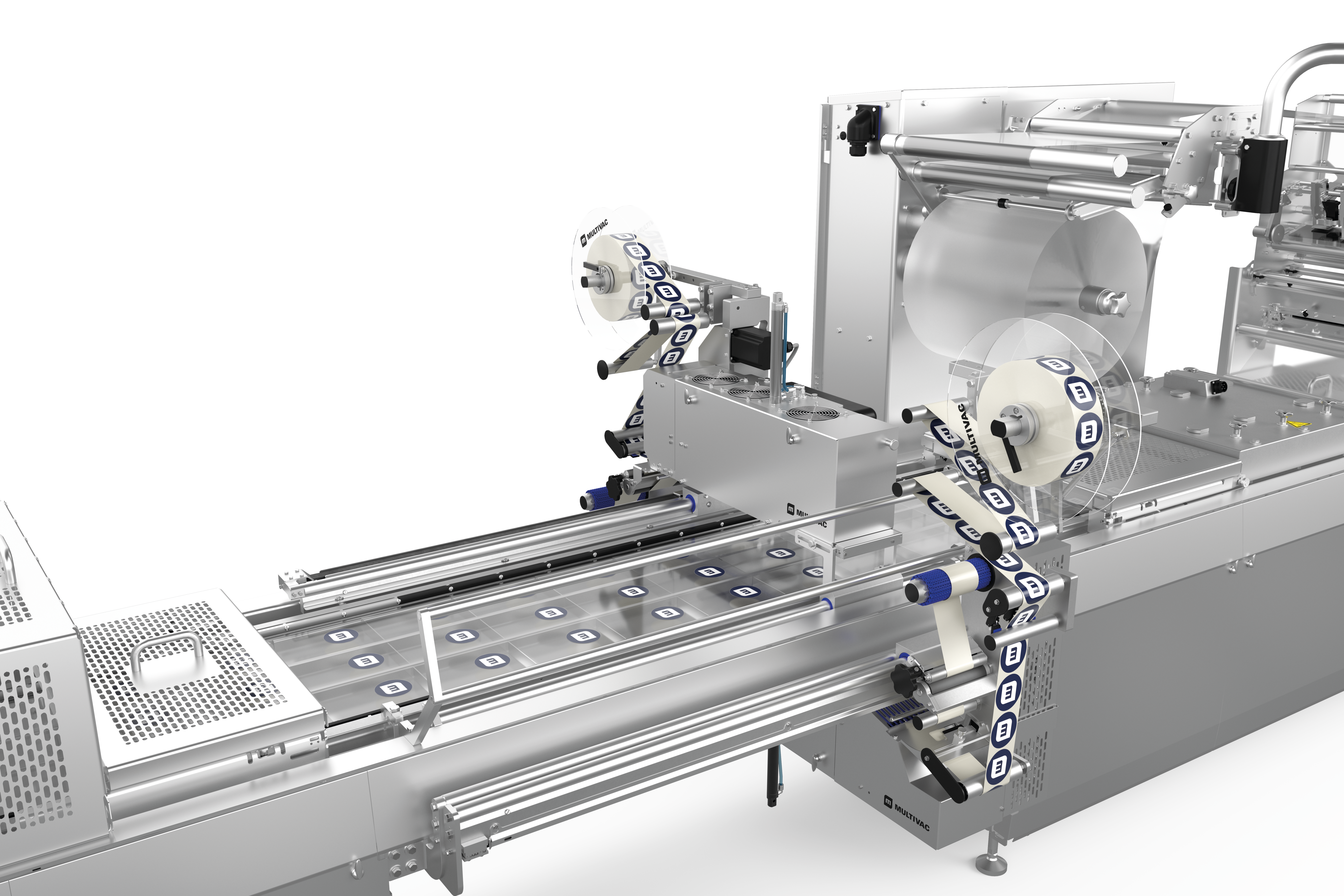
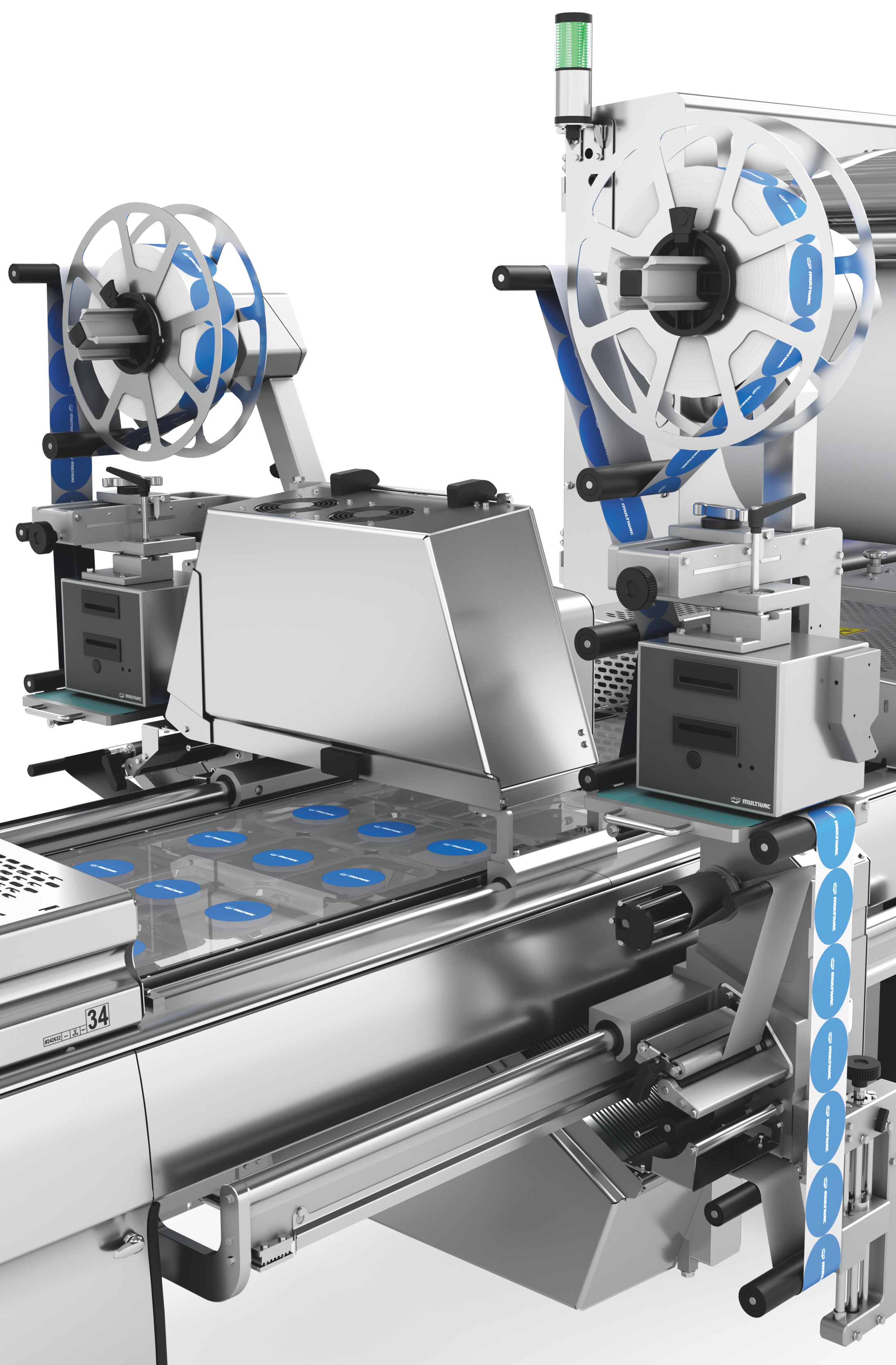
深絞り包装のラベリングと印字の柔軟性と信頼性
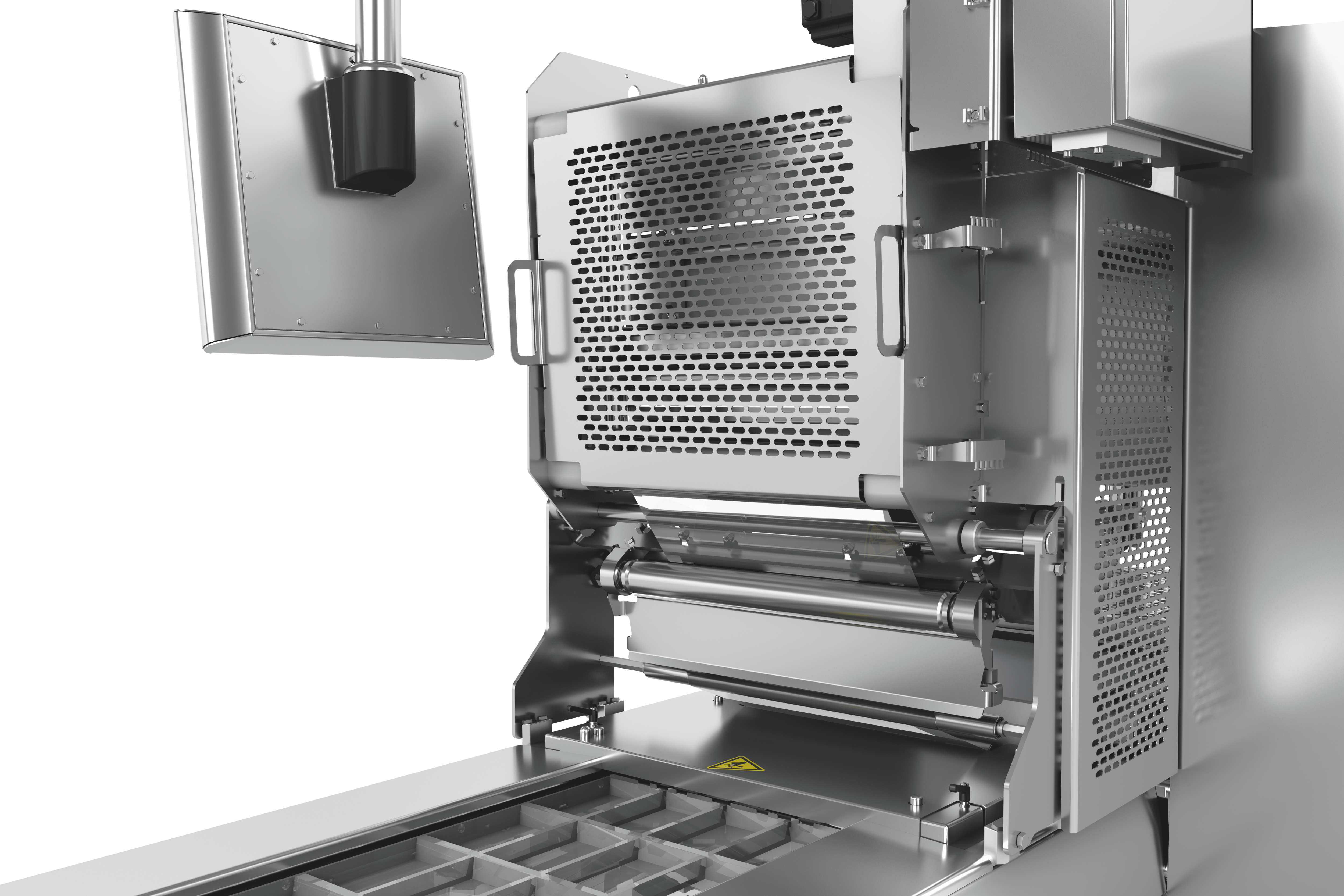
深絞り包装には、クロスウェブラベラーとダイレクトウェブプリンターによる、カスタマイズ可能で柔軟かつ完全に統合された2つのラベリングソリューションを提供します。ラベリングマシンとダイレクトウェブプリンターは、HMI(ヒューマン・マシン・インターフェース)により集中管理されています。フォーマットや素材の選択については、喜んでアドバイスさせていただきます。
効率と費用対効果
市場の要求は絶えず高まっています。企業は、将来にわたってその地位を維持するために、開発と市場の期待に追いつかなければなりません。経済的に実現可能な投資判断は、将来を見据えたものでなければなりません。これには、深絞り包装のラベリングも含まれます。例えば、長い交換時間は、装置の効率に悪影響を及ぼします。さらに、個々のラインコンポーネントが一つのまとまったユニットとして機能しなければ、プロセス全体が影響を受けることになります。
成功事例
 Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
Potato specialities securely packed and protected
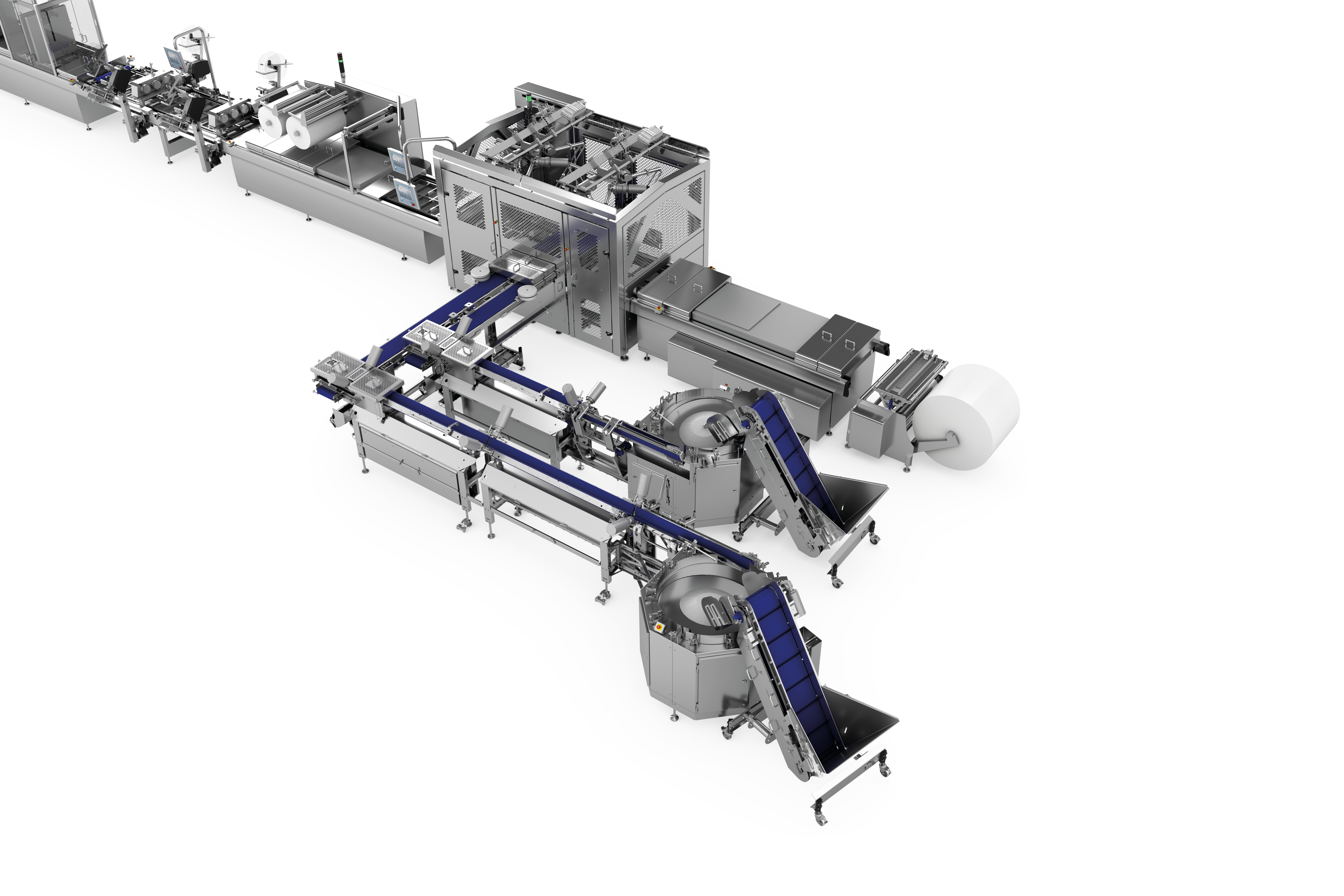
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
Mehr erfahren
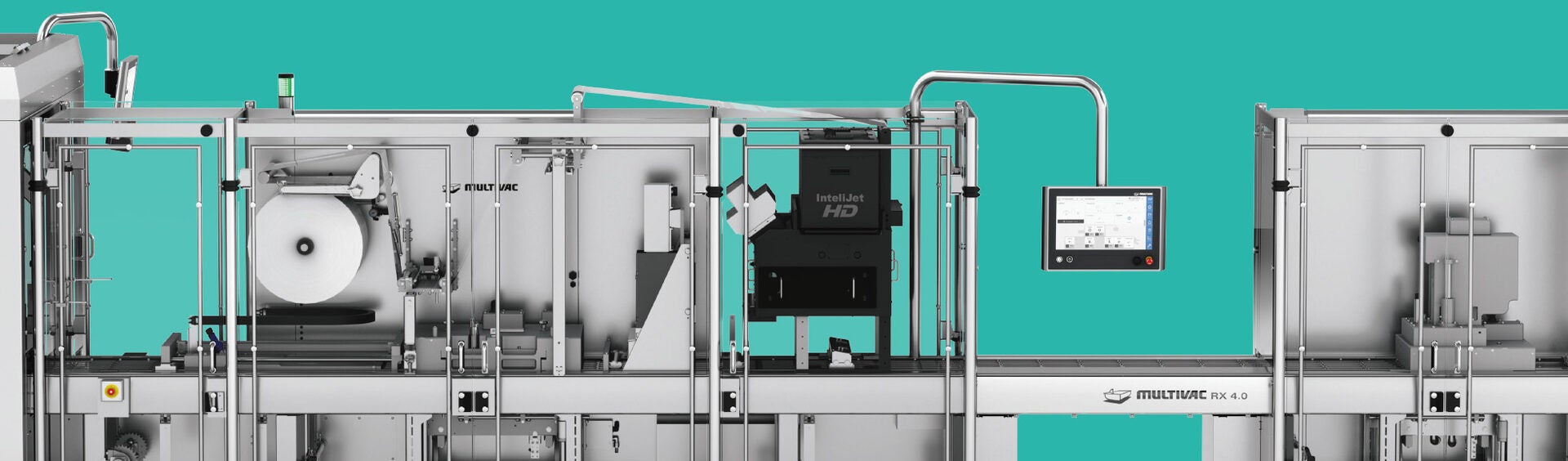
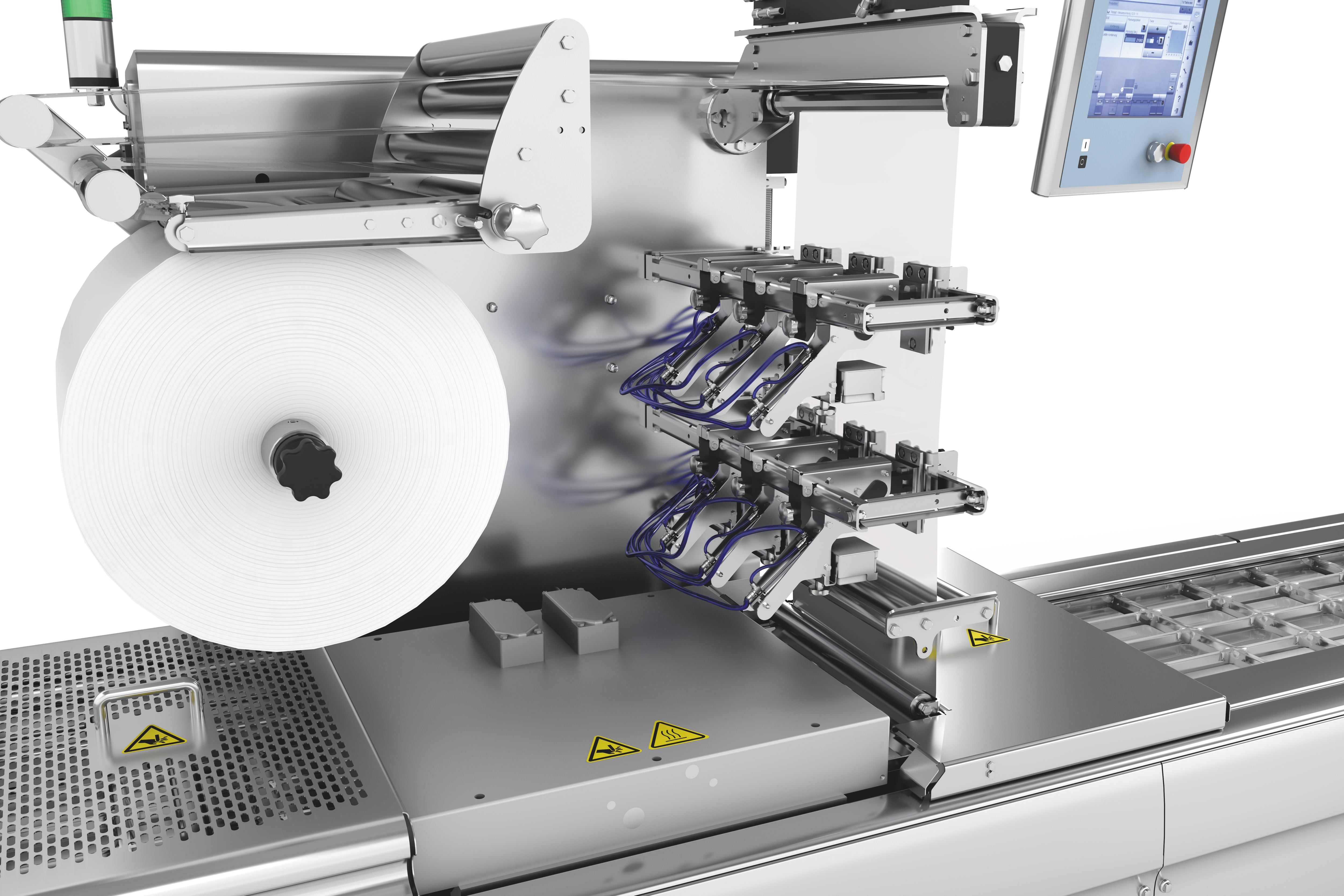
Digital printing systems fulfil challenging pack marking requirements
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
Mehr erfahren
 MULTIVAC Sausage Packaging Machine in Factory
MULTIVAC Sausage Packaging Machine in Factory