Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
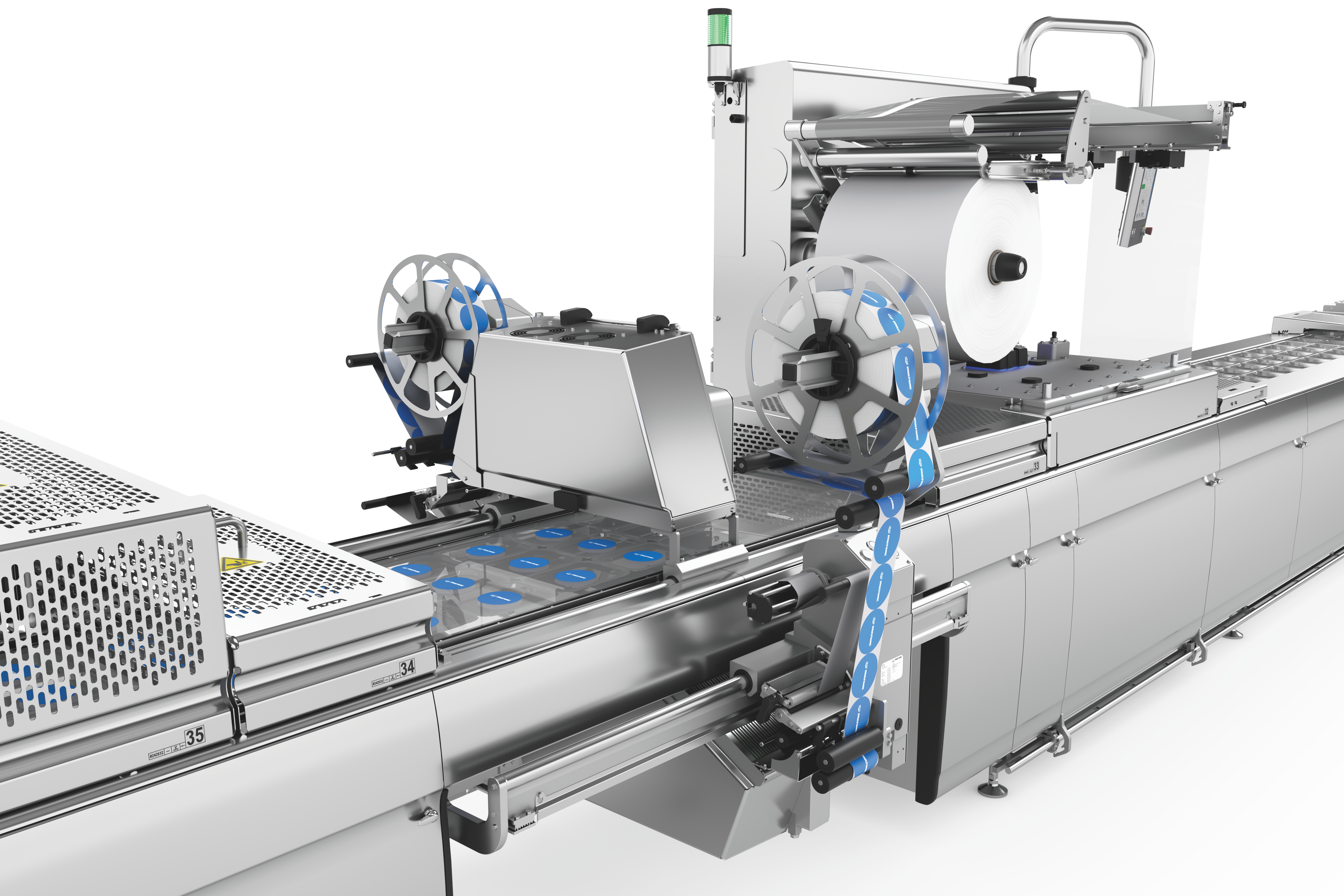
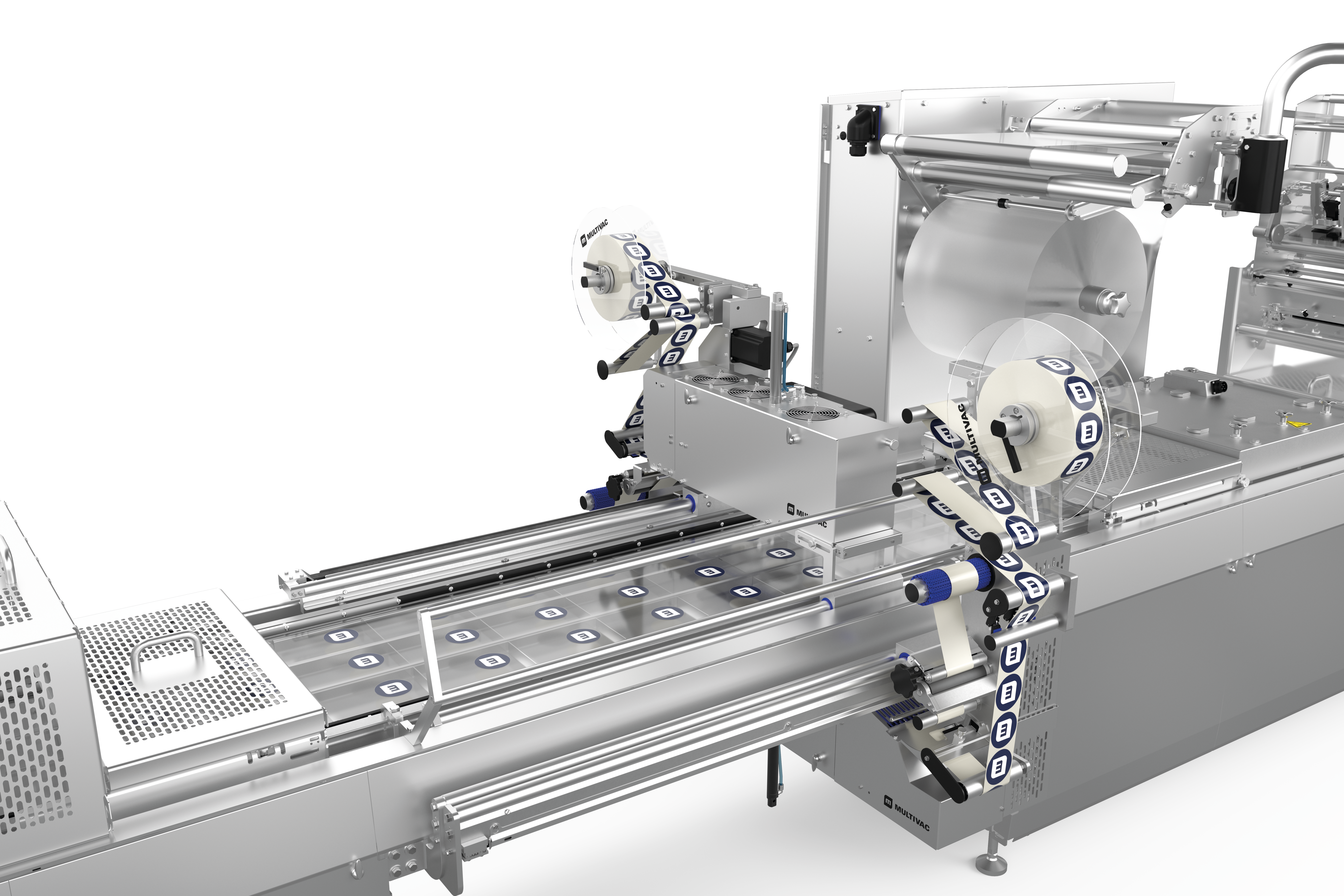
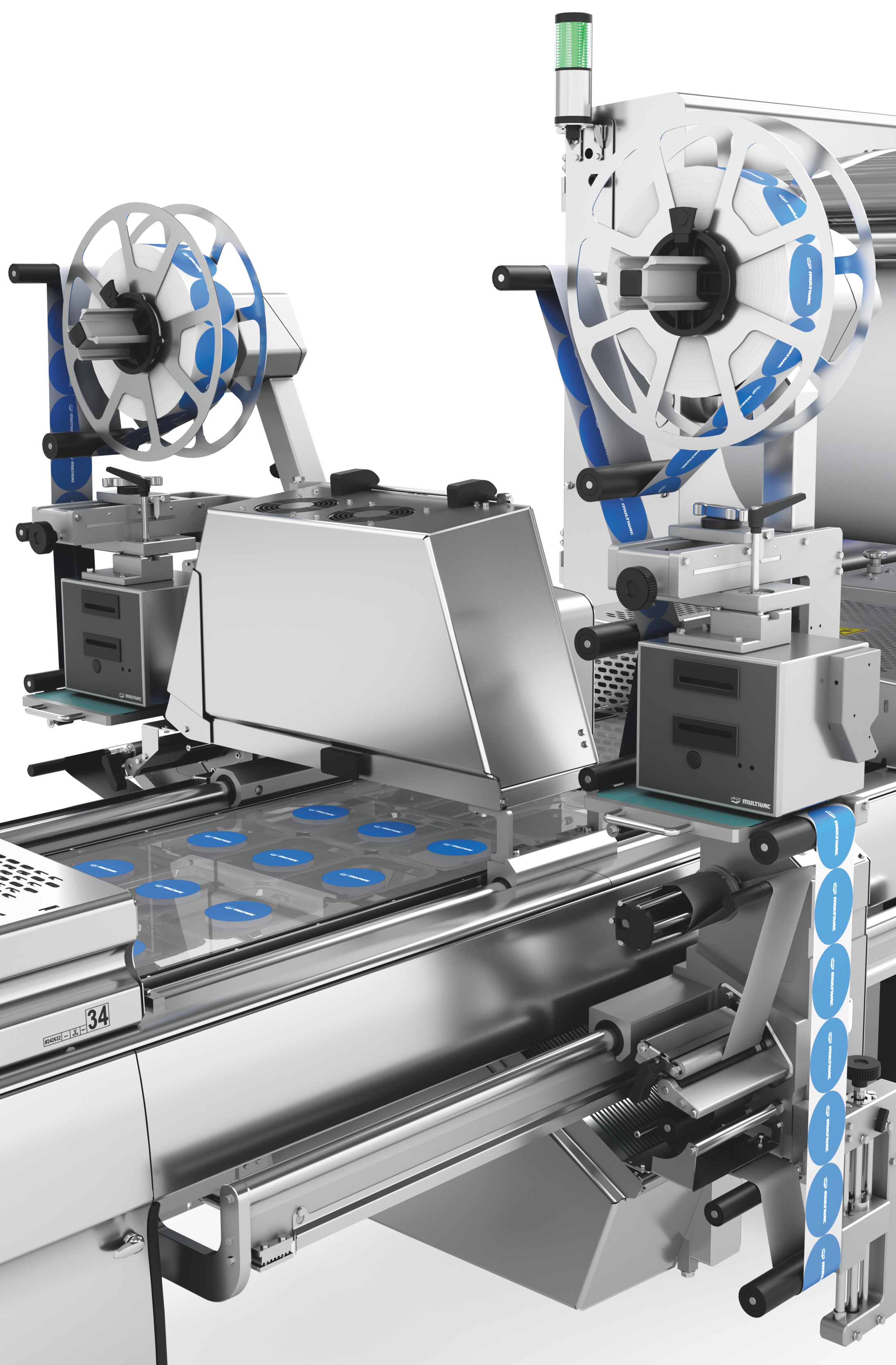
열성형 패키징 라벨링 시 유연성과 신뢰성
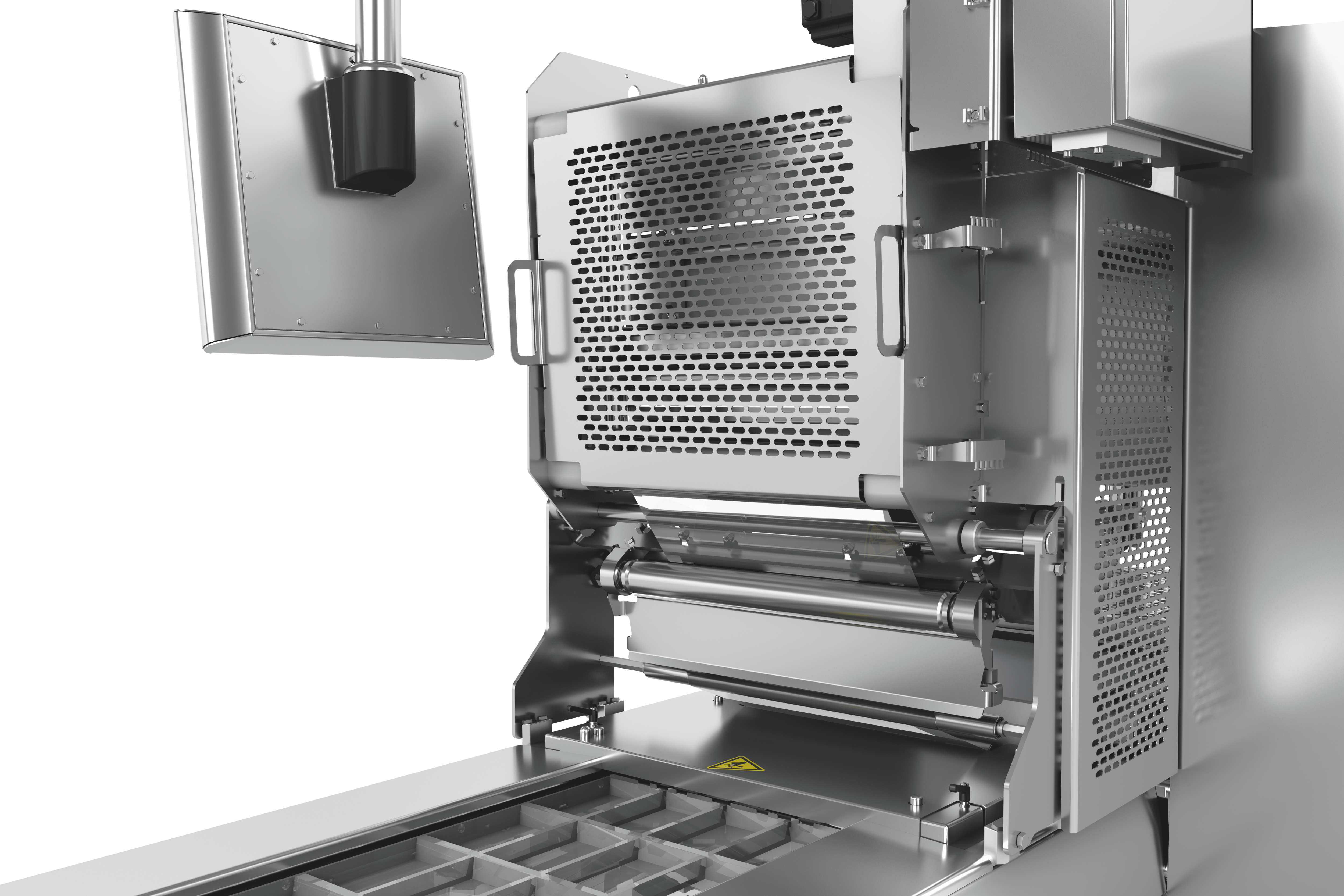
열성형 패키지 라벨링을 위해 당사는 크로스웹 라벨기와 다이렉트 프린터를 적용한 맞춤형, 유연하고 완벽하게 통합 가능한 라벨링 솔루션 두 가지를 제공합니다. 라벨링 장치와 다이렉트 프린터는 HMI(인간 인터페이스)를 통해 장비 메인 컨트롤에서 제어됩니다. 라벨 포맷과 재질 선택 시 상담을 해드립니다.
효율성과 경제성
시장 수요는 계속해서 증가하고 있으며, 기업은 미래에 자신의 위치를 유지하기 위해 개발 및 시장의 기대를 따라야합니다. 경제적으로 실행 가능한 투자 결정은 미래 경쟁력이 있어야 합니다. 열성형 패키징 라벨링도 포함됩니다. 예를 들어 교체시간은 장치 효율성에 부정적인 영향을 미칩니다. 또한 개별 라인 구성품이 개별 라인 구성 요소로 작동하지 않으면 전체 프로세스가 저하됩니다.
성공 사례
 Potato Harvesting Machine by MULTIVAC
Potato Harvesting Machine by MULTIVAC
Potato specialities securely packed and protected
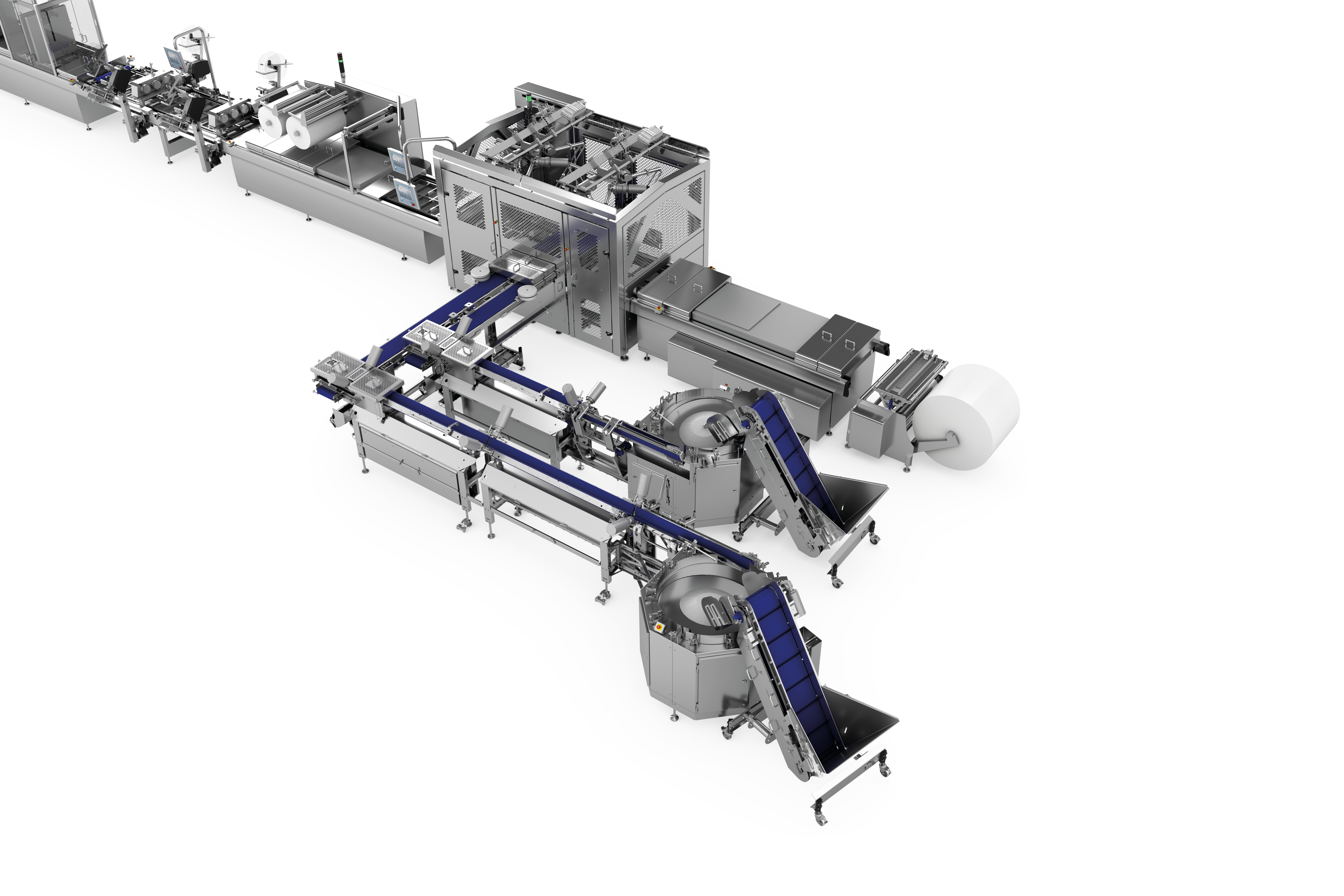
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
Systematic quality assurance from the growing of the product to the cooking process and right through to packing - that is the quality claim of Peka Kroef. The Dutch producer of pre-cooked potato specialities used the opportunity presented by the replacement of some of his packaging machinery to further improve the reliability and security of his overall packaging process. In conjunction with MULTIVAC, a solution was devised, which meant a major step forward in terms of productivity, efficiency, and sustainability.
추가 정보
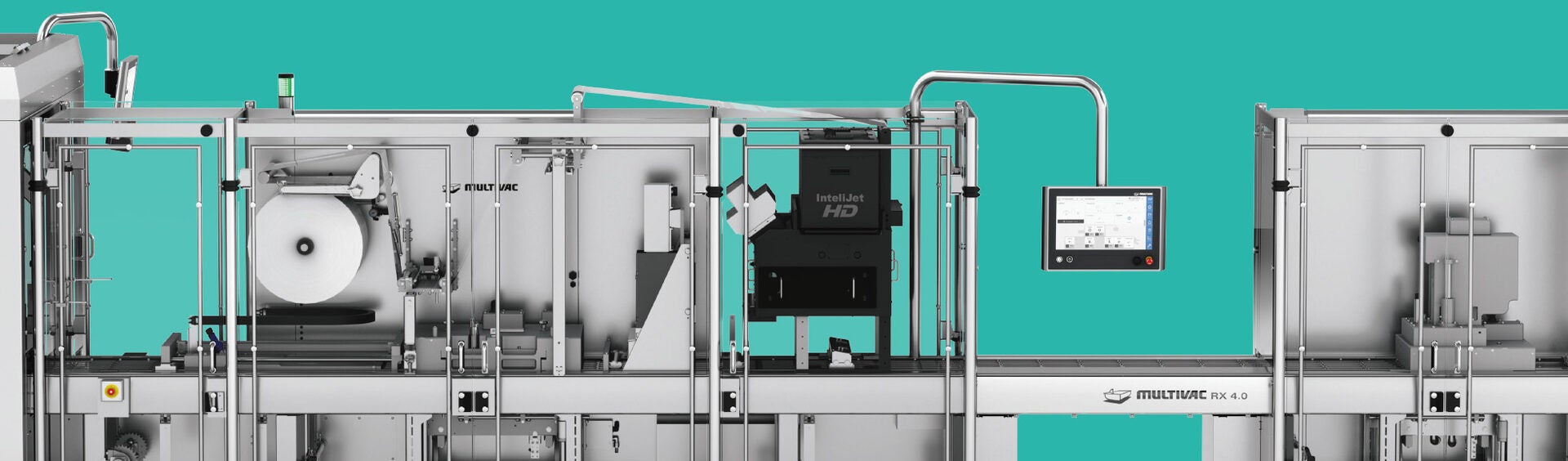
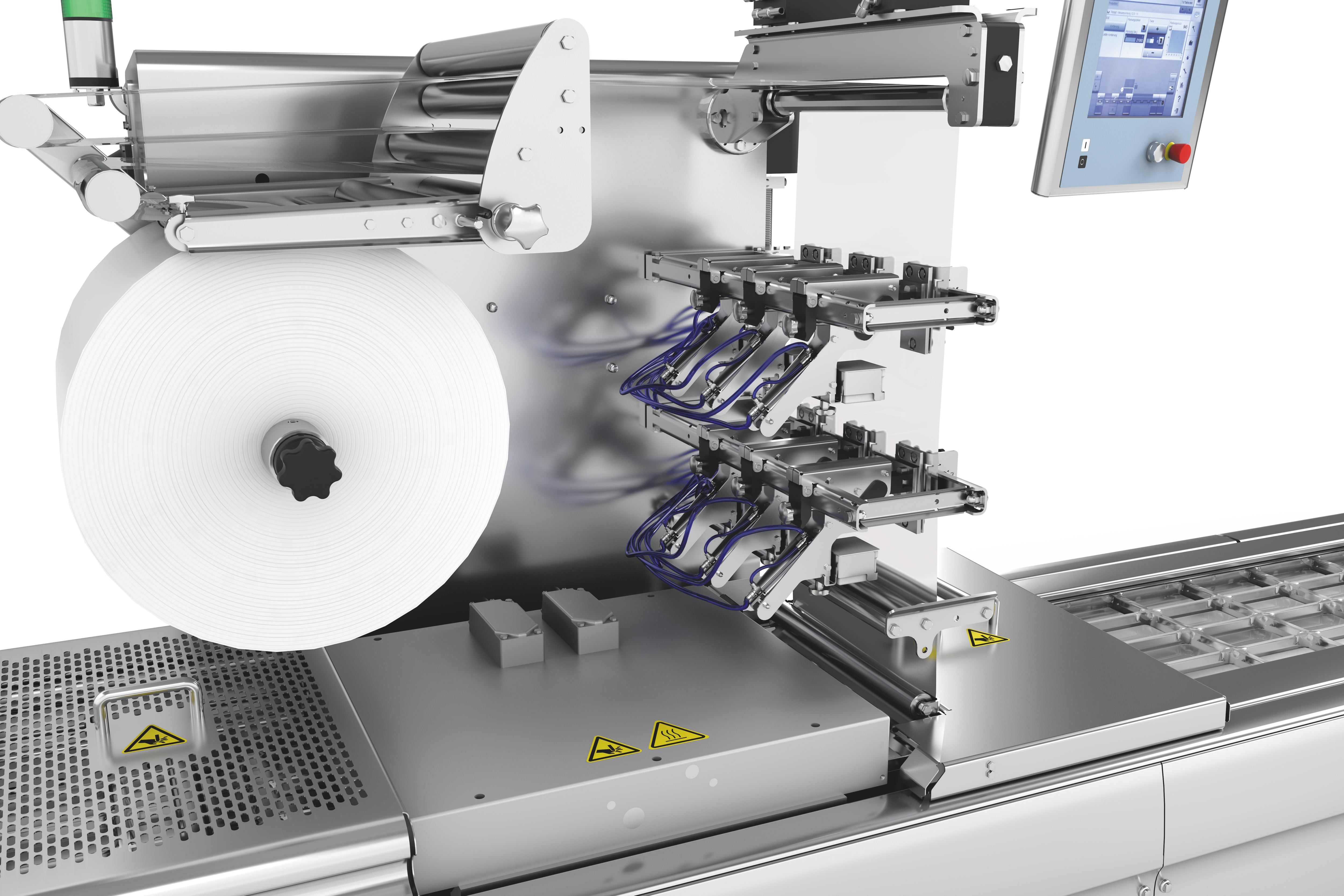
Digital printing systems fulfil challenging pack marking requirements
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
The UDI Directive applies to all companies, which manufacture medical products or bring these into circulation. From 25 April 2020 all products of Class III together with implants must be marked with a distinct and unique identification number, and this applies from May 2023 to products of Class IIa and IIb, as well as from 2025 to products of Class I. This distinct product identification is allocated by various bodies - these are currently GS1, HIBCC and ICCBBA. The products together with their master data and a so-called Basic UDI are registered in a new, central database (Eudamed) covering all of Europe.
추가 정보
 MULTIVAC Sausage Packaging Machine in Factory
MULTIVAC Sausage Packaging Machine in Factory