MULTIVAC packaging solutions for pharmaceutical products
Our priority is to provide in-depth consulting and develop a customized solution, working closely with your team, to ensure that the packaging meets your specific requirements.
Our custom applications cover biopharmaceuticals, blow-fill-seal products, auto-injectors, vials, ampoules, and pre-filled syringes.
We deliver reliable packaging solutions using state-of-the-art, GMP-compliant machinery designed to meet the strict standards of the pharmaceutical industry. Our systems support the flexible and efficient packaging of sensitive pharmaceutical and medical products across various formats, batch sizes, and configurations. Our solutions are scalable and adaptable to a diverse range of materials, thoroughly tailored to your requirements, whether you're creating single or combination packages.
Categories
Successfully implemented packaging solutions for pharmaceutical products
Our services for the pharmaceutical sector
MULTIVAC pharmaceutical packaging line solutions are designed to ensure full compliance with regulatory requirements while maintaining the highest efficiency and cost-effectiveness. Our engineering and technical support teams provide comprehensive assistance throughout the project lifecycle. In close collaboration with your team, we develop customized packaging solutions precisely aligned with your pharmaceutical product and process specifications.
Examples of applications for pharmaceutical products
- Packing in a natural atmosphere
- Modified atmosphere packaging (MAP)
- Packaging under vacuum
- Packing with skin film under vacuum
Complementary technologies
- Package Printing applications
- Labelling applications
- Inspection applications
- Complete line solutions integration
MULTIVAC solutions
Pharmaceutical Products
 MULTIVAC Packaging Machine Close-Up View
MULTIVAC Packaging Machine Close-Up View

 MULTIVAC T710 Packaging Machine Front View
MULTIVAC T710 Packaging Machine Front View
 MULTIVAC Packaging Machine VS500
MULTIVAC Packaging Machine VS500
 Automation Technology Interface by MULTIVAC
Automation Technology Interface by MULTIVAC
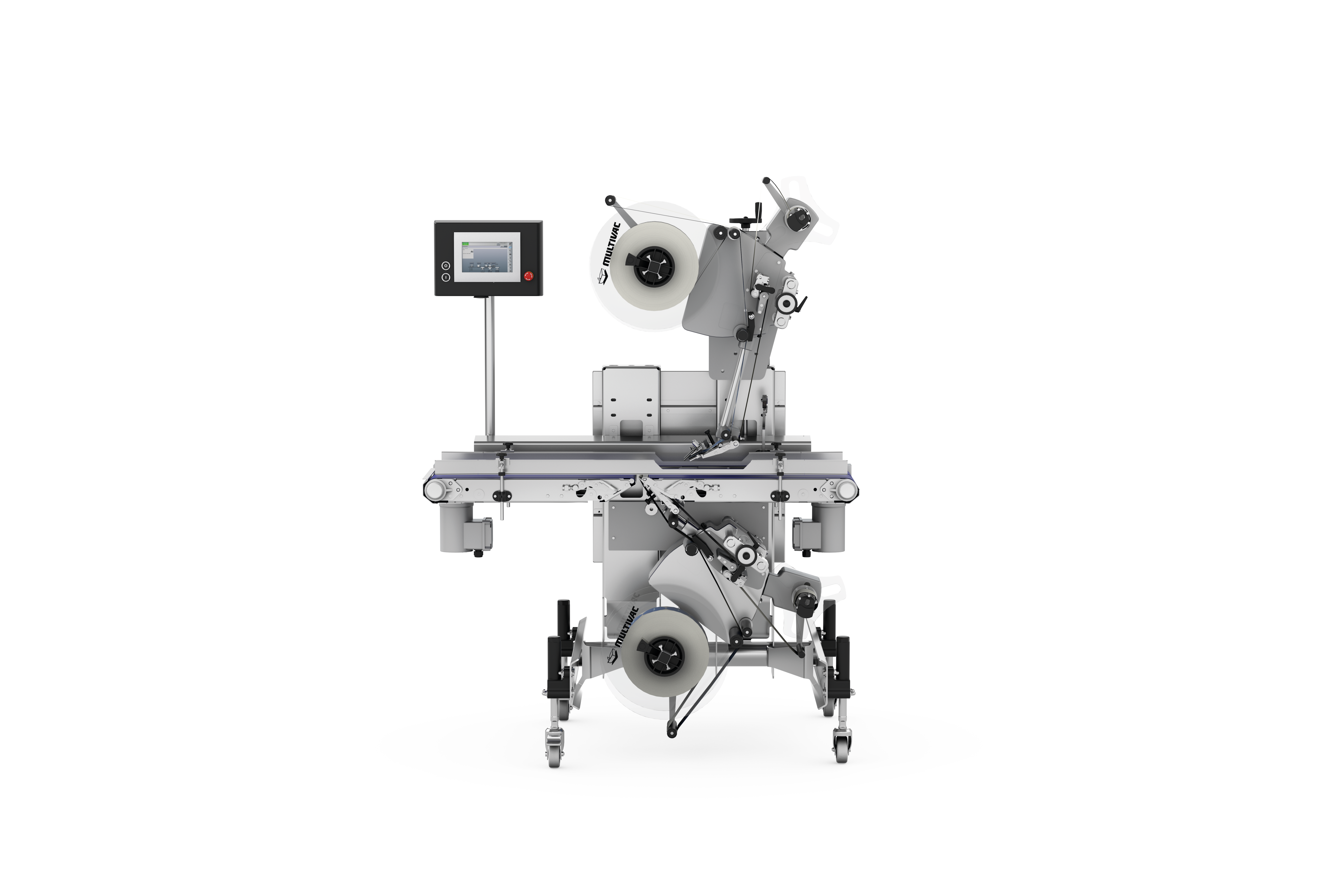

MULTIVAC line solutions for pharmaceutical products
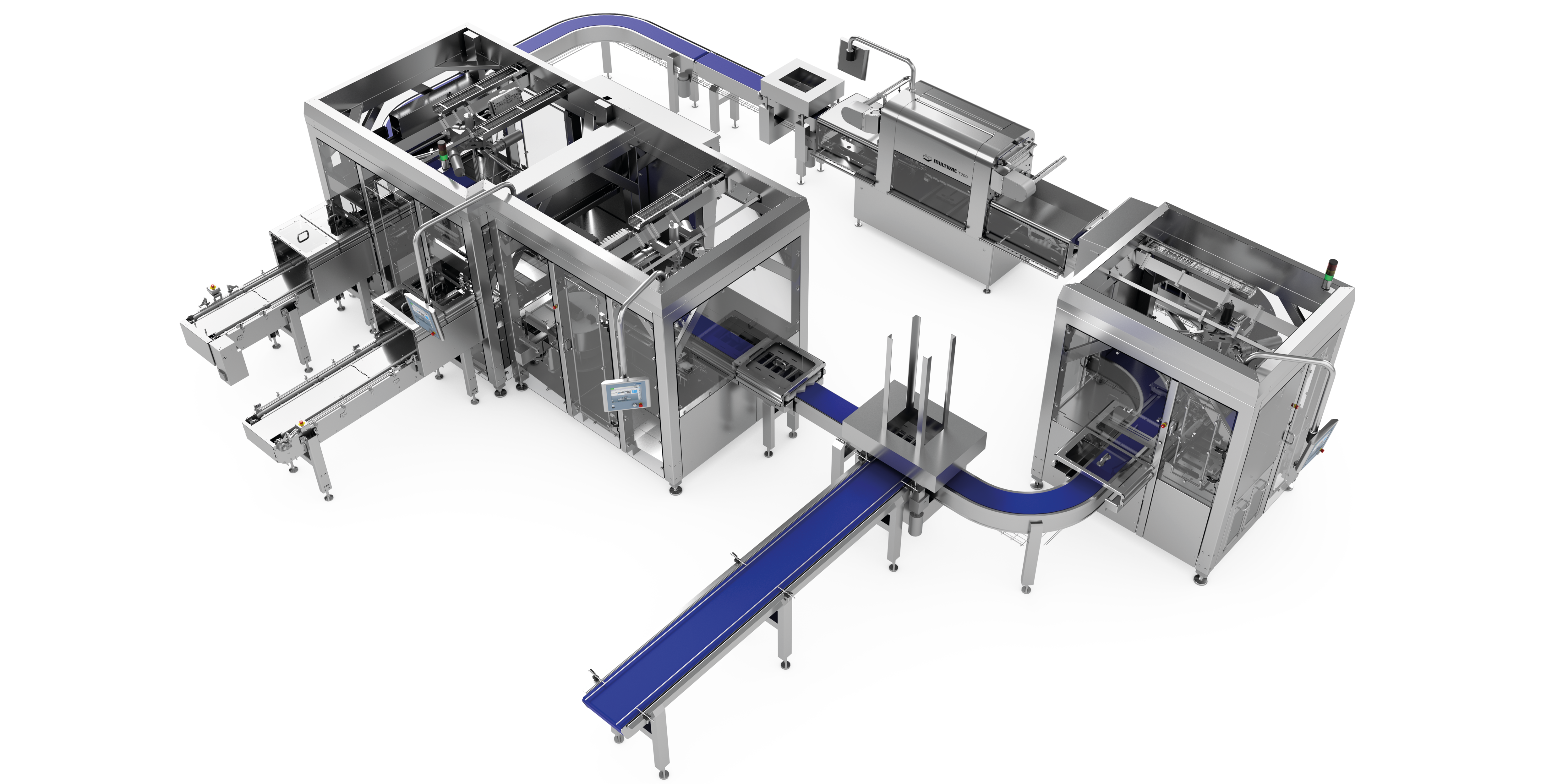 Example structure of a line solution for pharmaceutical products
Example structure of a line solution for pharmaceutical products
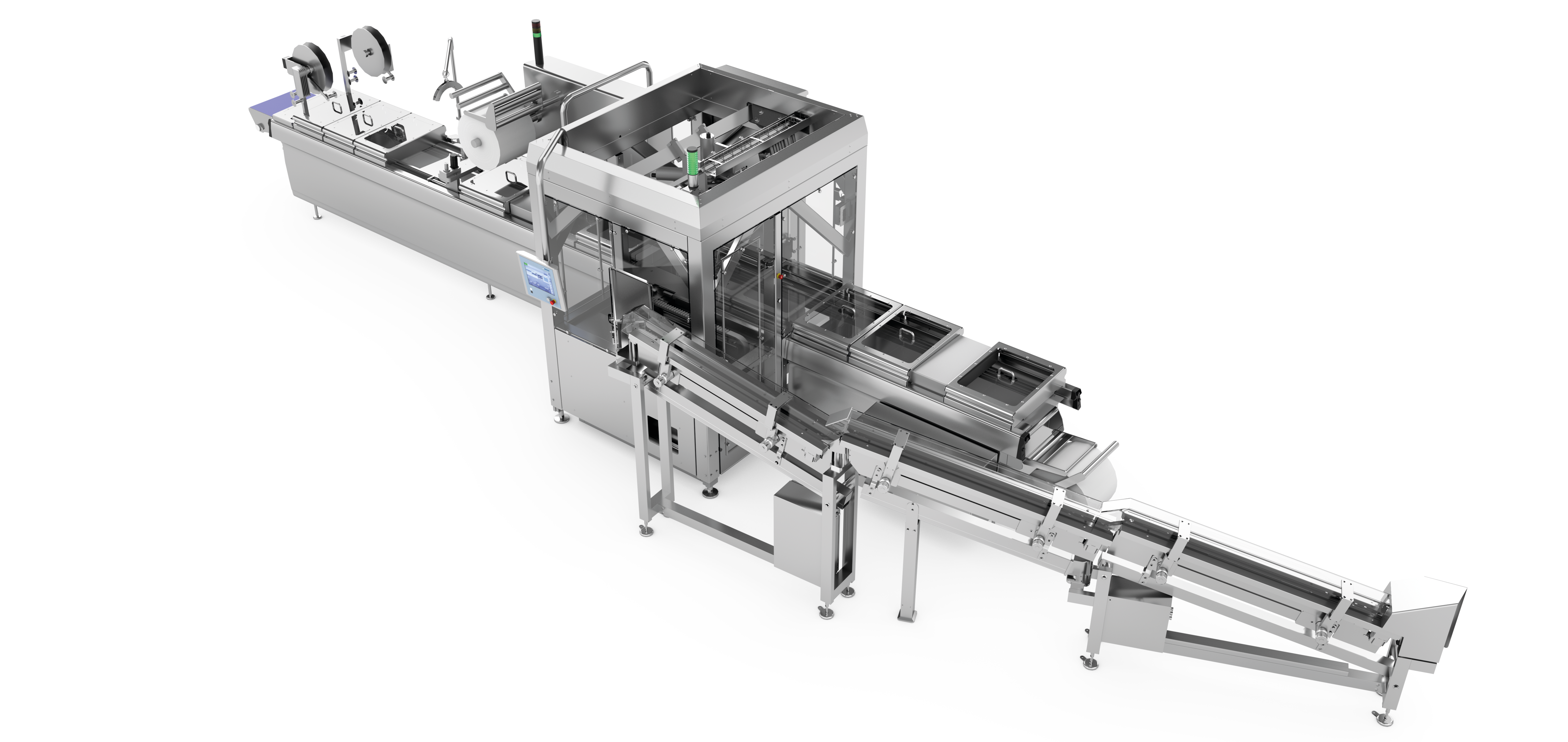 Example structure of a line solution for pharmaceutical products
Example structure of a line solution for pharmaceutical products
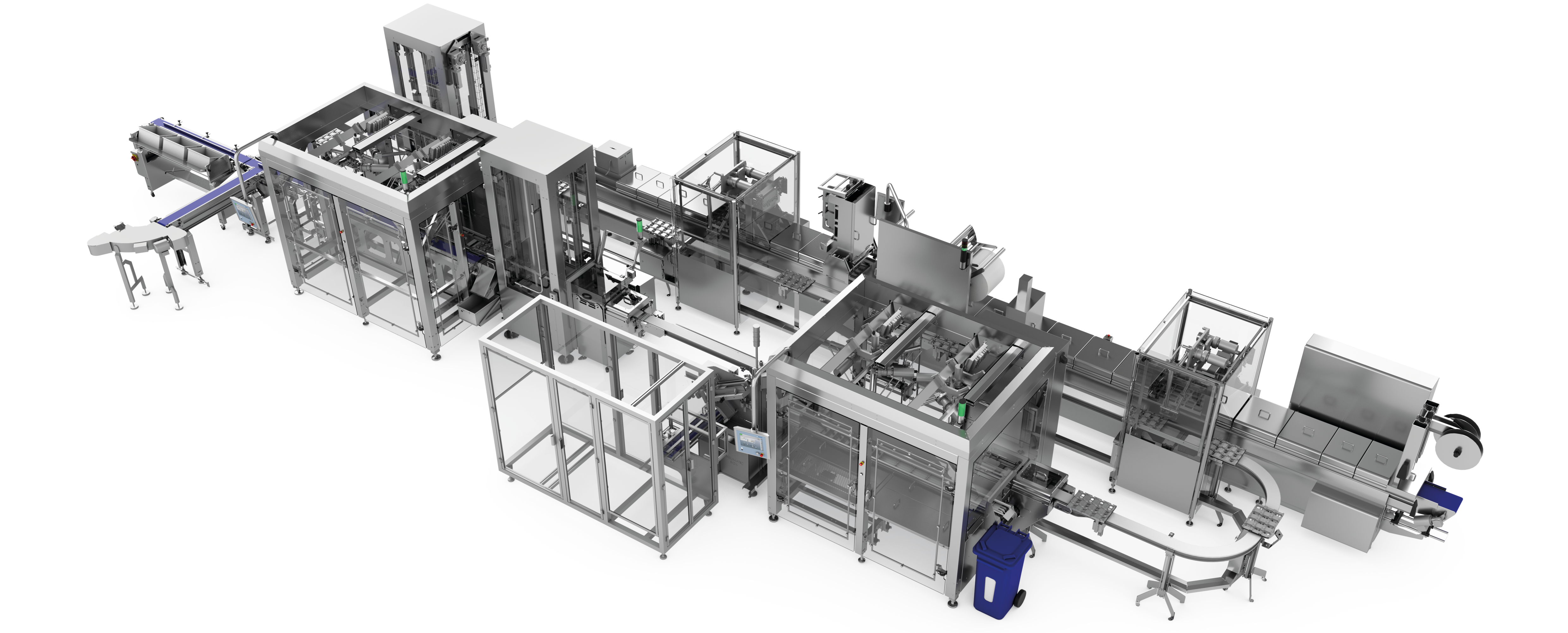 Example structure of a line solution for pharmaceutical products
Example structure of a line solution for pharmaceutical products
- Example structure of a line solution for pharmaceutical products
- Example structure of a line solution for pharmaceutical products
- Example structure of a line solution for pharmaceutical products












